LESSON 1
ACIDS:
An acid is a compound which when dissolved in water forms hydrogen ions as the only positively charged ions. It is the hydrogen ions that cause acidic properties and these are formed in the presence of water. Hydrogen ion is a proton because a hydrogen atom consists of a single proton and a single electron. The electron is lost when hydrogen ion is formed.
An acid turns blue litmus red and contains hydrogen ions which may be replaced, directly or indirectly, by a metal.
Examples of acids.
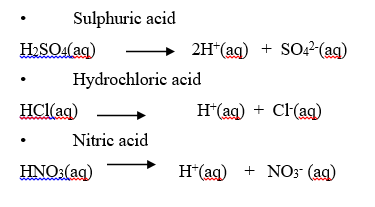
1. Mineral acids (common laboratory acids)
- Hydrochloric acid
- Sulphuric acid
- Nitric acid
The three acids are commonly used in the laboratory. They are sometimes called mineral acids because they were first prepared from certain minerals.
2. Organic acids
- Methanoic acid
- Ethanoic acid
- Citric acid
Common acids in every day life include: citric acid in lemons, tannic acid in tea, lactic acid in sour milk, tartaric acid in baking powder.
STRENGTH OF ACIDS
1. Strong acid
A strong acid is one that is completely ionized when in solution.
Examples
BASICITY OF AN ACID
Basicity of an acid is the number of hydrogen ions that can be formed when one mole of acid ionizes completely. Sulphuric acid is dibasic and phosphoric acid is tribasic.
Acid Basicity
HCl 1 Monobasic
HNO3 1 Monobasic
H2SO4 2 dibasic
H3PO4 3 Tribasic.
TEST FOR ACIDS
Acids are tested using substances called indicators. An indicator is a substance that has different colours in acid and alkaline solutions.
|
Indicator colour in acid |
colour in alkaline |
|
Litmus red |
blue |
|
Methyl Orange red |
yellow |
|
Phenolphthalein Colourless |
purple |
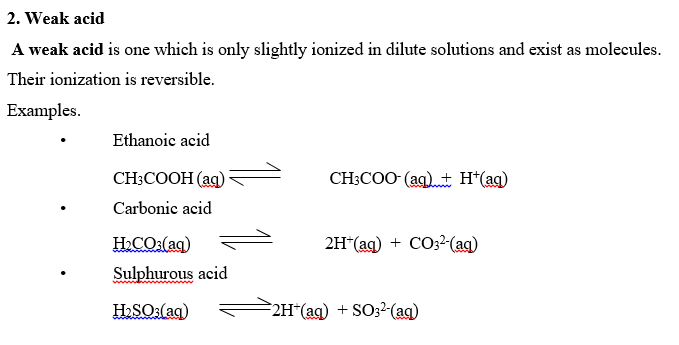
NB: Weak acids turn litmus pink.
