LESSON 2
INDICATORS
Indicators are substances which change colour and indicate the degree of acidity or alkalinity of solutions. Some simple indicators can be made from coloured substances which occur naturally.
Extraction of simple indicator
- Take some coloured plant material e.g. rose petals or red cabbage leaves.
- Crush the material with a mortar and add it to a small beaker.
- Cover the material with a mixture of water and ethanol (5:1 by volume).Warm the material and solvent by placing in a larger beaker containing hot water.
- Stir the hot mixture well for 10 minutes. Filter and collect the coloured filtrate.
- Add the made indicator to various acidic solutions, water and alkaline solutions. Indicators and pH scale
Universal indicator solutions are the most useful indicator. It shows a series of colour changes as the acidity or alkalinity of a solution changes.
Add 2 or 3 drops of universal indicator to test the various solutions.
The pH scale is a scale of numbers from 0 to 14 used to express the degree of acidity or alkalinity of a substance. The pH number of water and other neutral substances is 7.Acidic solutions have a pH number less than 7, as the number decreases acidity increases. Alkaline solutions have a pH number of 8-14.The increase in number indicates increasing alkalinity.
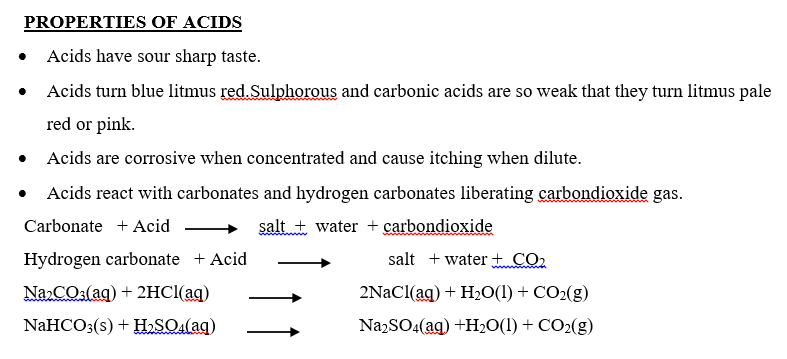
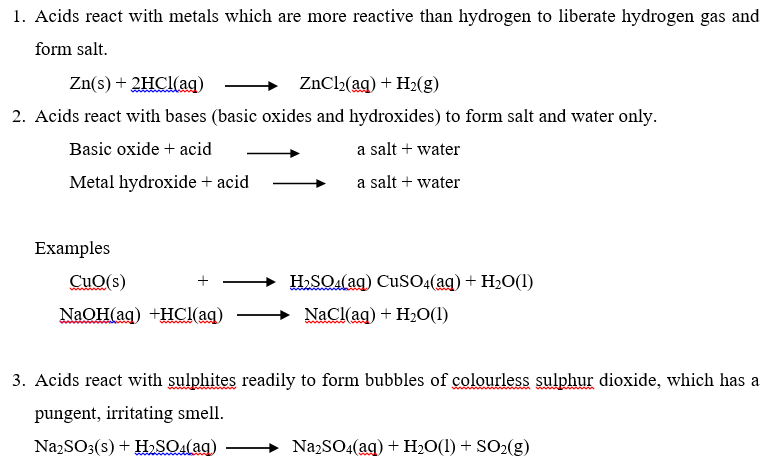
USES OF ACIDS
- Sulphuric acid is used in car batteries.
- Nitric acid is used in the manufacture of dyes, explosives and fertilizers.
- Hydrochloric acid is used in pickling metals.
- Ethanioc acid is used in seasoning of food salads.
