LESSON 3
BASES AND ALKALIS
BASE:
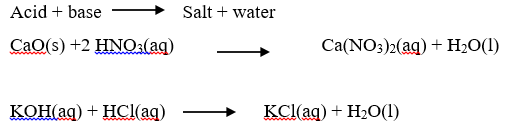
A base is a compound which contains oxide (O2-) or hydroxide (OH–) ions and reacts with an acid to form a salt and water only. Bases are oxides and hydroxides of metals. They neutralize acids forming a salt and water only. The reaction between bases and acids are called neutralizations.
Neutralization is a reaction between an acid and a base to form a salt and water only.
Examples of bases:
- Calcium oxide (CaO)
- Copper (II) oxide (CuO)
- Magnesium hydroxide Mg (OH)2)
- Sodium hydroxide (NaOH)
- Potassium hydroxide (KOH)
AN ALKALI
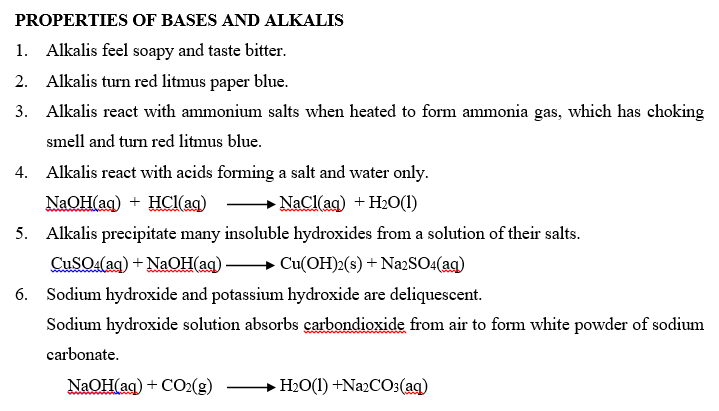
An alkali is a compound which when dissolved in water produces hydroxide ions (OH–) as the only negatively charged ions. Soluble bases are called alkalis. Therefore, all alkalis are bases, but not all bases are alkalis. An alkali is a base which is soluble in water. An alkaline solution is formed when a base dissolves in water.
Examples; Sodium hydroxide (NaOH), Potassium hydroxide (KOH), Calcium hydroxide[ Ca(OH)2]
Aqueous ammonia ( NH4OH)
STRENGTH OF ALKALIS
1. Strong alkalis
A strong alkali is one which is completely ionized in solution.
Examples. Sodium hydroxide, potassium hydroxide and calcium hydroxide.
Strong alkalis are very good conductors of electricity.
2. Weak alkalis
A weak alkali is one which ionizes only partially in solution. It’s characterized by reversible ionization. A weak alkali has low conductivity of electricity.
Example
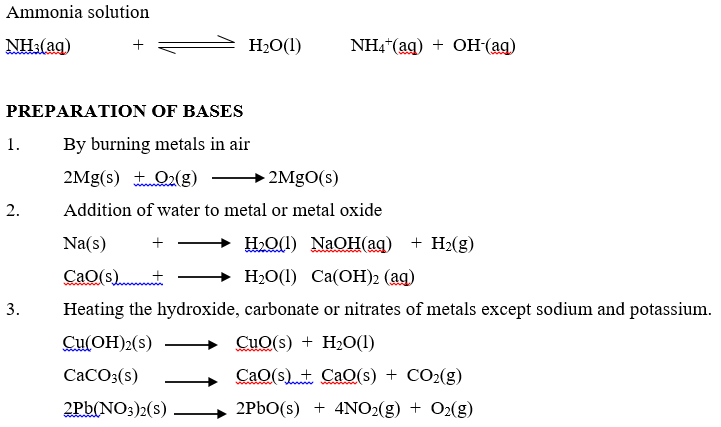
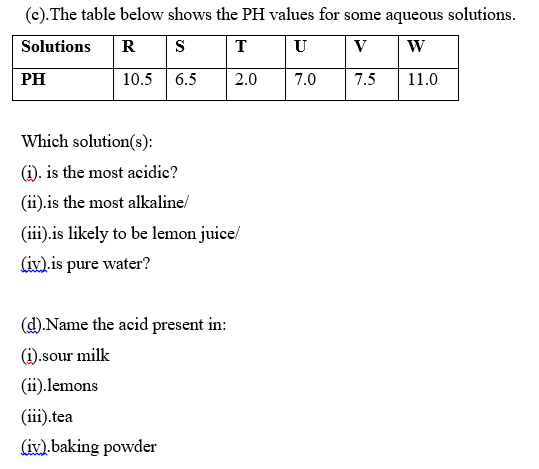
USES OF ALKALIS AND BASES
1. Sodium hydroxide and potassium hydroxide are used in the manufacture of soap.
2. Calcium oxide is used:
(i). In building industry as slaked time, in mortar and plaster.
(ii). In the manufacturing of bleaching powder.
(iii). As a drying agent to dry ammonia and ethanol.
3. Magnesium hydroxide is used as antacid to neutralize acidity in the stomach.
Exercise
1. Giving examples in each case write equations for the reaction of sulphuric acid with:
- a metal
- a metal oxide
- a metal hydroxide
- a metal carbonate
- a metal hydrogen carbonate.
2. (a).What is an acid?
(b).State three physical properties of acids.
(c).Write the name and formula of the 3common mineral acids used in the laboratory.
(d).Write chemical equations to iilustrate the properties of acids using hydrochloric acid and the following compounds; (i).Copper(II) oxide
(ii).Magnesium metal
(iii).Potassium carbonate solution
(iv).potassium hydrogen carbonate solution.
3.(a).Define the terms;
(i).Base
(ii).Alkali
(iii).Neutralization reaction
(b).Give two examples of;
(i).Bases
(ii).Alkalis
4.(a).What is an indicator?
(b).Describe an experiment to prepare an indicator in the laboratory from either coloured flower petals or red cabbage leaves.