LESSON 5
PREPARATION OF SALTS
Salts are prepared basing on their solubility in water. A salt is either soluble when it dissolves in water or insoluble when it does not dissolve in water.
Solubility of salts. Some salts can dissolve in water readily. Other salts are insoluble in water.
The solubility of a salt is the amount of the salt that is required to saturate 100g of water at a particular temperature.
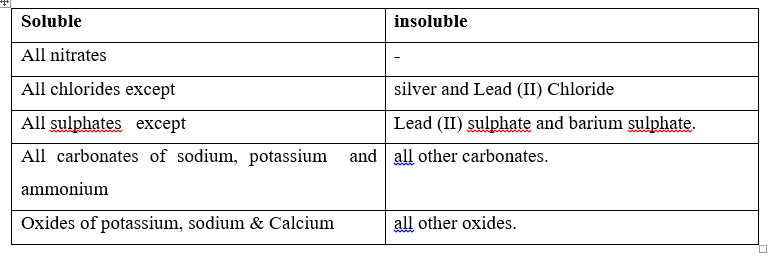
Note: Oxides are not salts but also classified as soluble and insoluble.
Oxides are used in the preparation of salts. Soluble salts are prepared in solution and crystallized from saturated solution. Soluble salts are prepared by the action of an acid on:
|
(i). |
a metal |
|
(ii). |
A hydroxide of a metal |
|
(iii). |
An oxide of a metal (basic oxides) |
|
(iv). |
A carbonate of a metal |
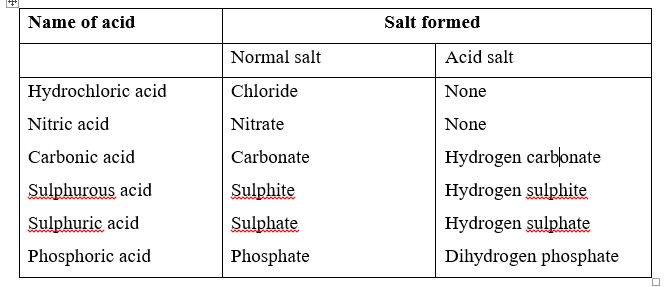
The kind of salt to be prepared will depend on the kind of acid used.
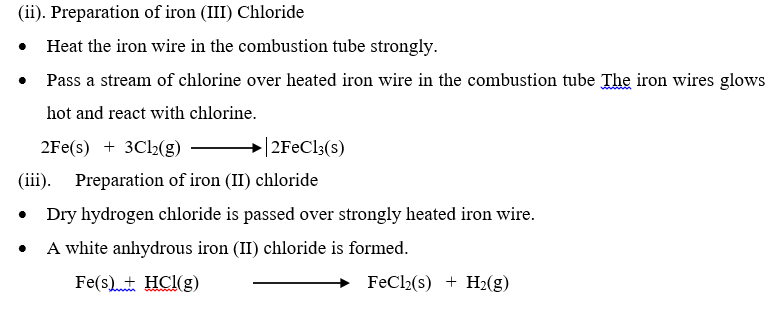
(a). Action of acid on a metal.
Preparation of Zinc sulphate crystals from zinc metal.
- Pour dilute sulphuric acid in a beaker
- Add Zinc granules until in excess and add copper (II) sulphate solution to speed up the reaction. Effervescence occurs.
- When the reaction stops, filter the excess Zinc. Evaporate the filtrate by heating in evaporating dish until crystallization point. Dip a glass rod in the solution and check if crystals appear in it or pour a little hot solution in a test tube and cool under tap shaking all the time.
- Wash the crystals 2-3 times with little cold distilled water and dry them by pressing gently between two filter papers.

(b). Action of acid on alkali
Preparation of sodium chloride from sodium hydroxide by neutralization.
- Pipette 25cm3 of sodium hydroxide into a conical flask and add 2-3 drops of phenolphthalein indicator.
- Fill the burette with hydrochloric acid solution .
- Run the acid from burette into sodium hydroxide in a conical flask. Shake constantly until the solution turns colourless.
- Transfer the solution into evaporating dish and evaporate until its concentrated.
- Pour the concentrated solution in a beaker and allow to cool and crystallize.
- Wash the crystals with little distilled water and dry between filter paper.
Sodium sulphate can be prepared in the same way.

(c) Action of acid on insoluble basic oxide
Preparation of copper (II) sulphate from copper (II) oxide.
- Put dilute sulphuric acid into a beaker and warm gently.
- Add copper (II) oxide to the warm acid little at a time .Continue to add the oxide until no more will react, showing all the acid has been neutralized.
- Filter off excess copper (II) oxide and collect a blue copper (II) sulphate filtrate.
- Evaporate the filtrate until crystallization point. Dip a glass rod into the hot solution to check if crystals form on it. If crystals form, stop heating and allow it to cool and crystallize.
- Wash the crystals with little distilled cold water and dry them between filter papers.

(d). Action of acid on insoluble carbonate.
- Preparation of Lead (II) nitrate crystals from lead carbonate.
- Pour dilute nitric acid in a glass beaker
- Add Lead (II) carbonate little at a time while stirring, effervescence occurs.Continue to add until no more will react showing that all the acid has been neutralized.
- Filter off excess lead carbonate and collect a colourless filtrate of Lead (II) nitrate.
- Evaporate the filtrate until crystallization point. Dip a glass rod into the hot solution to check if crystals form on it. If crystals form, stop heating and leave to cool and crystallize.
- Wash the crystals with little cold distilled water and dry between filter papers.
Preparation of insoluble salts.
Insoluble salts are prepared by a method called double decomposition or precipitation. Double decomposition means that the two compounds used in the reaction as reactants both decompose to form new compounds.
Precipitation is the act of throwing down a solid (precipitate) when two aqueous solutions are mixed.
Preparation of Lead (II) Sulphate
- Add dilute sulphuric acid to Lead (II) nitrate solution into glass beaker, stirring all the time.
- Filter off the precipitate and pour off the liquid.
- Wash the precipitate 2-3 times with hot distilled water to remove any acid.
- Dry the precipitate in a steam oven or by leaving it in the air.
Direct combination of elements (Direct synthesis)
(i) Preparation of Iron (II) Sulphide
- Put three spatula endful of powder sulphur to iron filings in a test tube.
- Add three spatula endful of powered sulphur to iron filings in a test tube.
- Heat the mixture strongly in Bunsen flame until no further change.
A red glow spreads through the whole mixture.