TOPICAL ASSESSMENT
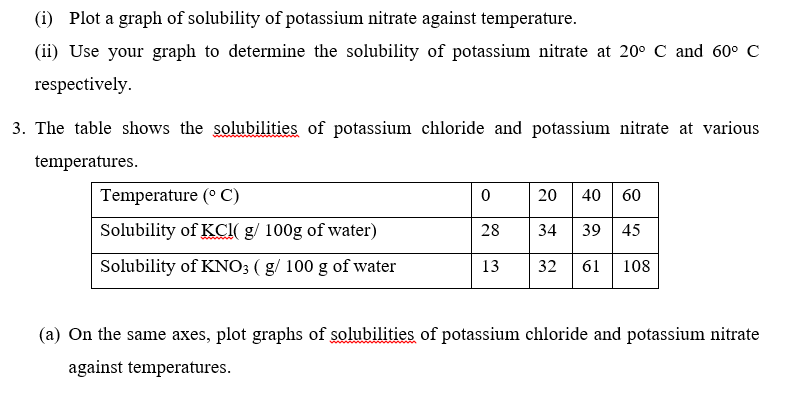
(b) Determine the temperature at which the solubilities of the two salts are equal.
(c) Which of the two salts dissolves more rapidly with increase in temperature?
(d) State what would happen if a saturated solution of potassium chloride at 40 o C was cooled. (e) A saturated solution of potassium chloride at 40o C was cooled to 35o C .Calculate the mass of potassium chloride crystals formed.
4. (a) Explain the following terms:
- Solute
- Super saturated solution
(b) A saturated solution of sodium nitrate was made at 25o C .Use the following data to determine the solubility of sodium nitrate at 25oC.
Mass of evaporating dish = 58.05g
Mass of evaporating dish + salt solution= 123.53g
Mass of evaporating dish + dry salt = 61.28
5. (a) Explain giving example(s) what is meant by
(i) Basicity of an acid.
(ii) An acid salt.
(b) Outline how a pure dry sample of sodium hydrogen sulphate can be prepared in the laboratory.
(c) An acidified solution of barium chloride was added to aqueous sodium hydrogen suphate.State what was observed and write equation for the reaction that took place.
6. Define the following terms
- Saturated solution
- Crystallization (c ) Neutralization
- Precipitation
- Describe how pure lead (II) sulphate can be prepared in the laboratory starting from lead nitrate.
7. (a) Define the terms
- A normal salt
- An acid salt
- Basic salt
(b) Give one example of
- Normal salt
- An acid salt
- Basic salt
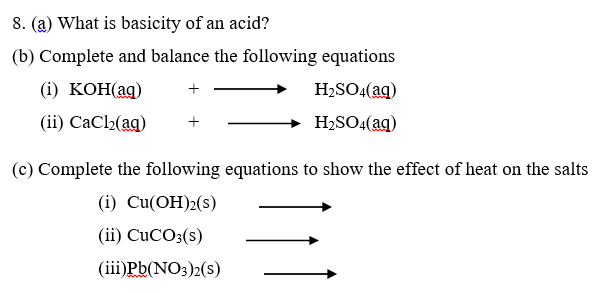
(a) Sulphuric acid is a strong acid.
- Explain what strong acid means.
- Write equation to show how sulphuric acid ionizes in water.
(b) Write equations for the reactions that occur when dilute sulphuric acid is added to
- Zinc carbonate
- Sodium hydroxide solution.
- Magnesium ribbon
10. (a) Give the name and formula of
- a dibasic acid
- Amphoteric hydroxide
- Amphoteric oxide
(b) Write equation for the reaction of the acid named with
(i) An amphoteric hydroxide
(ii) An amphoteric oxide
(c ) Salts can be prepared by the following methods:
- Neutralization of an acid by base
- Action of a metal on an acid
- Double decomposition
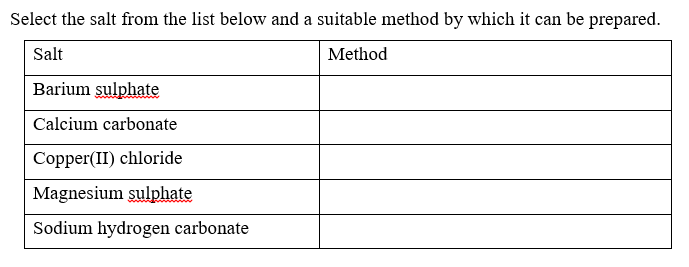
10. (a).Name four general methods of preparing salts from dilute acids and name one salt prepared from each method.
(b) .Write an equation in each case to show how the acid is used to prepare the salt.
11. Sulphur acid is a strong dibasic acid.
(a) Explain the terms strong acid, and basicity.
(b) Dilute sulphuric acid was added to a solution of lead (II) nitrate.
(i) State what was observed.
(ii) Write an equation for the reaction.
12.(a) What is a salt?
(b) What are the products of the reaction between acid and a base?
(c) Give three characteristics of a base and three characteristics of acid.
13.(a) Explain the following terms:
(i) Double decomposition
(ii) Precipitation
(b) Write ionic equations for the reaction between hydrochloric acid and
- Sodium carbonate solution
- Sodium hydroxide solution
