LESSON 2
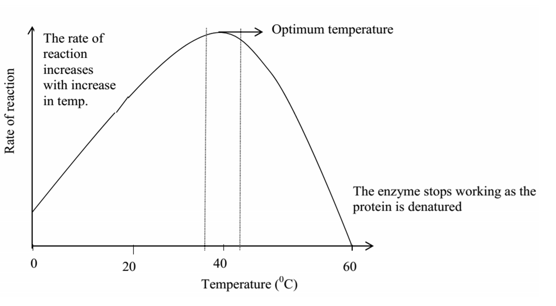
Enzymes work best at optional temperatures of (approximately 370 C). At very low temperatures, the rate of enzyme reaction is very slow because the enzyme is inactive at such low temperatures.
As the temperatures increase, the rate of reaction also increases gradually until it attains a peak where it has maximum activity and this always correspond at optimal temperatures. An optimal temperature is which promotes maximum enzyme activity. However with further increase in temperature, the rate of reaction decreases exponentially, sharply, steeply since at high temperatures, the enzyme is denatured ie the active site of the enzyme which is (protein in nature) is a altered (changed) or completely destroyed.
Graph showing the variation of enzyme activity with temperature
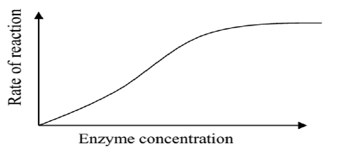
As the concentration of the enzymes increases, the rate of reaction also increases until all the substrates are being acted upon when the rate finally becomes constant.
A graph showing variation of enzyme activity with enzyme concentration
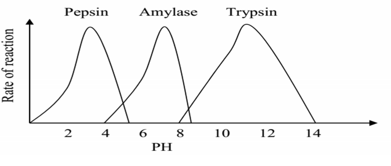
Enzyme reactivity is reduced or stopped completely if placed in a medium ` whose PH is different from that in which it works best (optimum PH).
PH varies slightly above or below an enzyme’s optimum PH resulting `in a marked fall in the enzyme efficiency. E.g. pepsin enzyme in the human stomach has a maximum activity with in acidic pH of 1.5 and 2.5 while the enzymes in the duodenum e.g. trypsin work at maximum with in alkaline pH of 8.5 to 9.5.
Graph showing variation of different enzyme activity with PH
2.3 Presence of enzyme inhibitors
Enzyme activities decrease in presence of enzyme inhibitors and increase in their absence.
Enzyme activators increase with presence of enzyme activators and decrease with absence of enzyme activators.
2.5 Mechanism of enzyme action
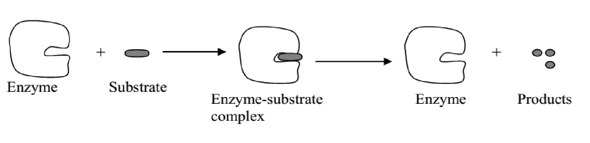
The widely accepted mechanism by which enzymes are known to work is the “key and lock” hypothesis.
The hypothesis suggests that the enzyme has a specific region known as the active site where the substrate fits like a key fits in a lock. The substrate must have a complementally shape to the active site of the enzyme. In this hypothesis the key is analogous to the substrate and the lock to the enzyme.
When the substrate enzyme activity with PH. combines with the enzyme, an enzyme- substrate complex is formed. This breaks down to release the products and the enzyme, which can pick other substrates.
Illustration