LESSON 7
SOLUBILITY AND SOLUBILITY CURVES
Solubility of a solute in a solvent at a particular temperature is the mass of a solute in grams required to saturate 100g of solvent at that temperature.
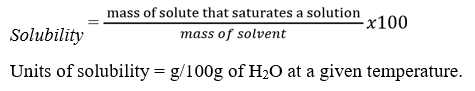
Definitions.
- An unsaturated solution is one which can take up more solute at that temperature.
- A saturated solution is one which cannot dissolve any more solute at that temperature in the presence of undissolved solute.
- A super saturated solution is a solution which contains more solute than is required to form a saturated solution at a particular temperature.
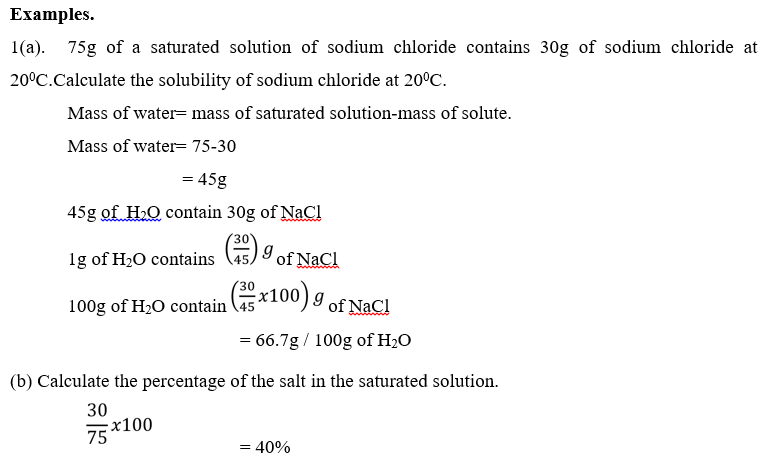
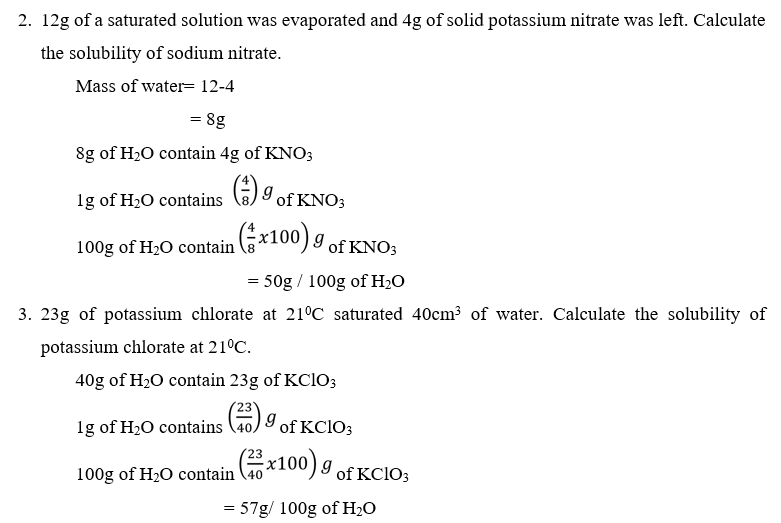
Experiment: To determine the solubility of potassium nitrate
- Potassium nitrate is dissolved in water to form a saturated solution.
- The solution in the beaker is allowed to settle so as to allow undissolved solid to separate from the liquid.
- Weigh the evaporating dish.
- The solution is transferred to a weighed evaporating dish and the mass of the solution and dish recorded.
- Solution is evaporated to dryness on a water bath to avoid loss of solid. The solid is allowed to cool and the dish and the solid reweighed. Treatment of results.
|
Mass of dish |
=a g |
|
Mass of dish+ solution |
=b g |
|
Mass of dish + salt |
=c g |
|
Mass of solution |
= (b-a) g |
|
Mass of salt |
=(c-a) g |
|
Mass of water |
= (b-c)g |
|
(b-c)g of water contain |
= (c-a ) |
SOLUBILITY CURVES.
The solubility curve of a substance is a graph showing how its solubility varies with temperature. The solubility of some salts eg KNO3, KClO3 increase with temperature and their curves rise steeply.
The solubility of some salts like NaCl, KCl change little with rise in temperature and their curves rise gently.
Some compounds like CaSO4 are less soluble in hot and cold water, their solubility curves fall gently.
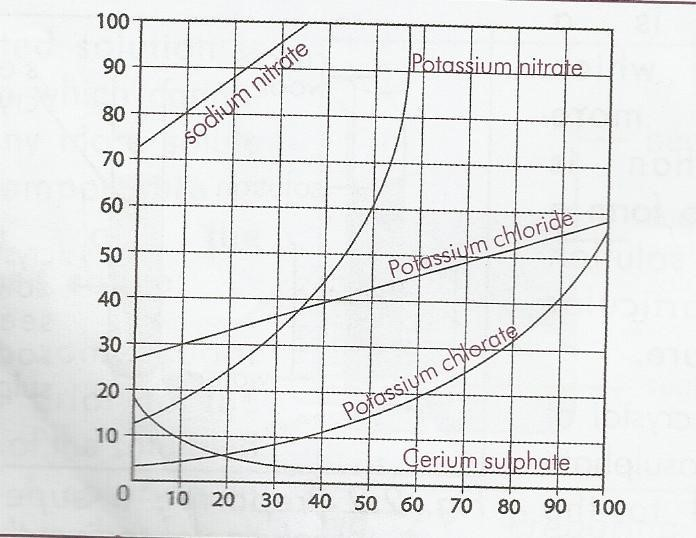
Variation is solubility of salt with temperature is the basis of fractional crystallization of salts. Fractional crystallization is used in extraction of sodium carbonate and sodium chloride.
Uses of solubility curve
- To obtain solubility of the salt at various temperatures.
- To determine the temperature at which a certain mass of salt when dissolved in water,can form a saturated solution.
- To calculate mass of salt precipitated by cooling from higher temperature to a lower temperature.
Applications of solubility
- Production of fizzy drinks eg soda which contain carbon dioxide dissolved at high pressure and low temperatures.On opening the bottle,the gas bubbles escape out of the solution.
- Extraction of sodium chloride from sea water by fractional crystallization.
- Purification of salts by fractional crystallization.
