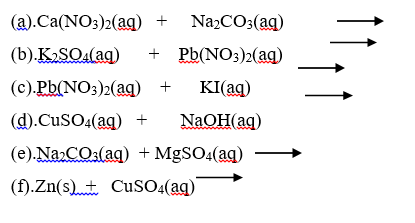LESSON FOUR
2. (a).Define the following terms:
(i).Valency (ii).Atom
(iii).Radical (iv).Molecule
(v).Element
(b). Using symbols, valency of radical and elements, write the chemical formulae for the following compounds:
|
1. Iron (II) sulphate |
2.iron (III) sulphate |
|
3. Calcium nitrate |
4.Aluminium sulphate |
|
5. Ammonium phosphate |
6. Aluminium oxide |
|
7. Lead nitrate |
8. Potassium carbonate |
|
9. Calcium hydrogen carbonate |
10. Magnesium nitride |
|
11. Sodium oxide |
12. Calcium phosphate |
|
13. Sodium hydrogen sulphate |
14. Aluminium chloride |
|
15. Calcium hydroxide |
16. Sodium sulphite |
|
17. Carbon dioxide |
18. Sodium sulphate |
(c).Give the chemical names for the following compounds:
(i).Cu(NO3)2 (ii).AlCl3
(iii).Fe(OH)3(iv) (iv).Mg(HCO3)2
(v).NaOH (vi). CO
(vii).SO2 (viii).H2SO4
(ix). (NH4)2CO3 (x).AgCl
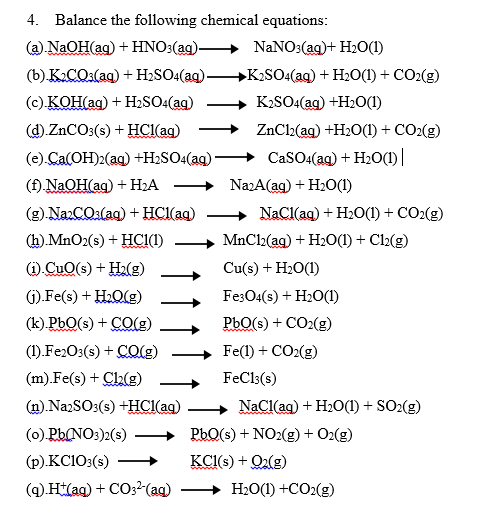
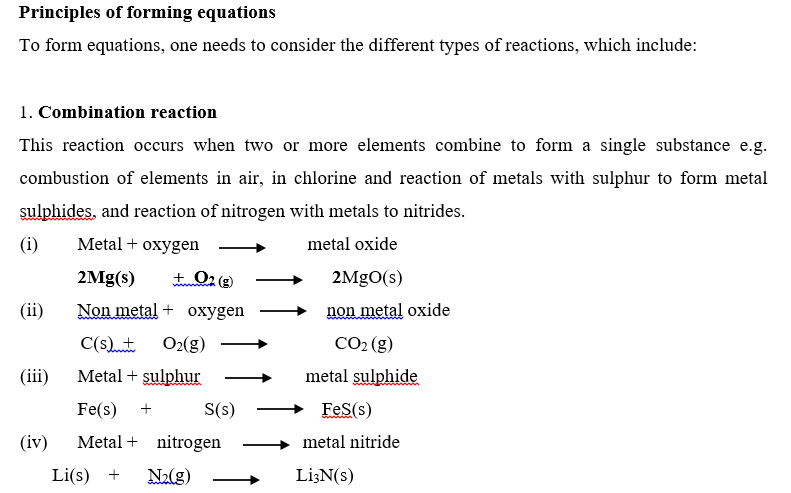
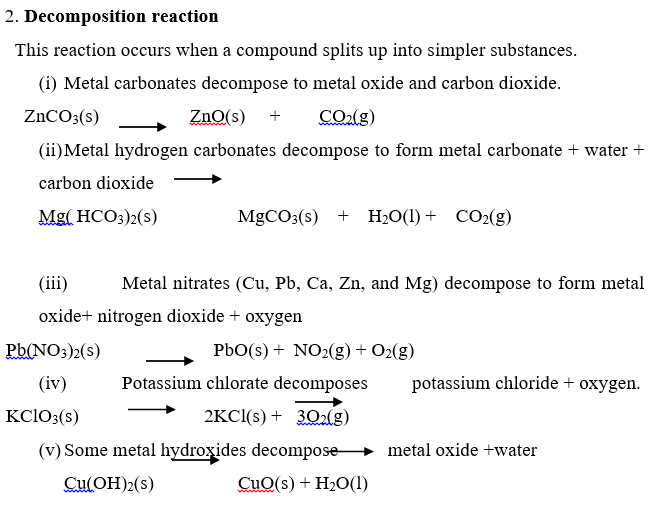
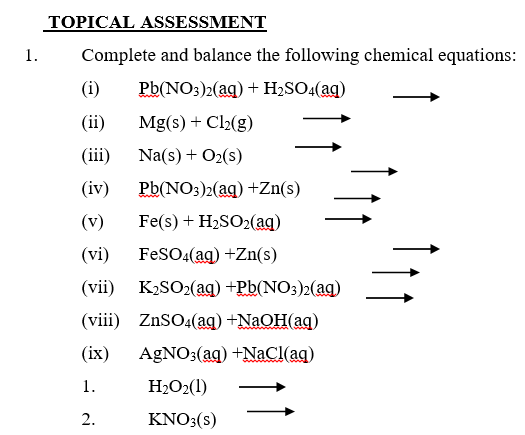
Complete and balance the following equations: