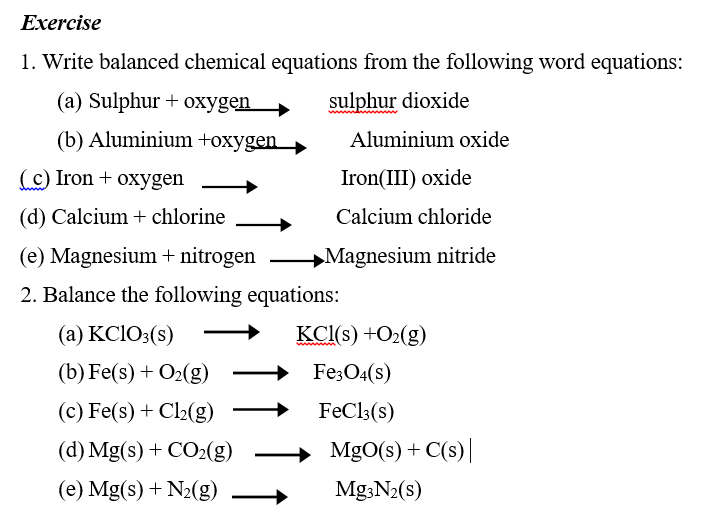
LESSON THREE
CHEMICAL EQUATIONS
A chemical equation represents a chemical change or reaction by means of symbols and formulae.
A word equation is written using the chemical names of the substances in the reaction.
Example: Magnesium burns in oxygen to form magnesium oxide
State symbols
State symbol for solid is (s)
State symbol for liquid (l)
State symbol for gas (g)
State symbol for an aqueous solution (aq).
Rules for writing Balanced chemical equations.
- On the left hand side, write formulae or symbols of reactants, on the right write formulae of products.
- Write a word equation for the reaction
- Balance the equation by writing small whole numbers in front of one or more formulae.
The process of making the number and kind of atoms equal on both sides of an equation is called Balancing equation
Equations
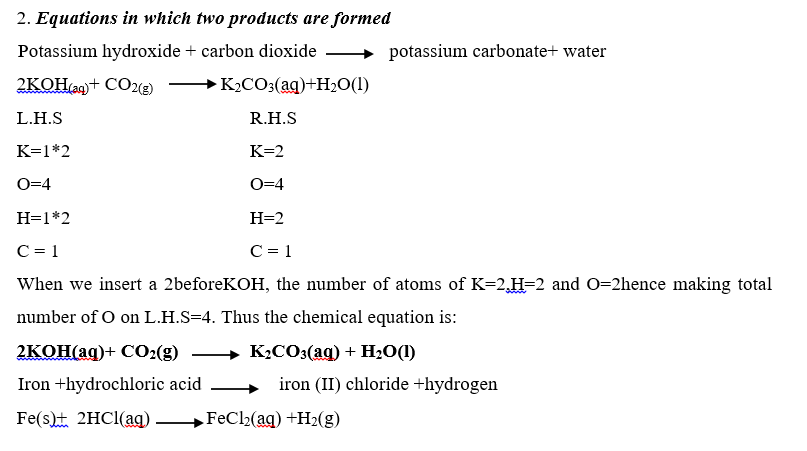
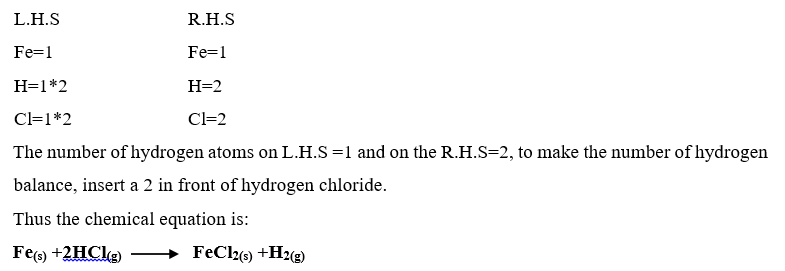
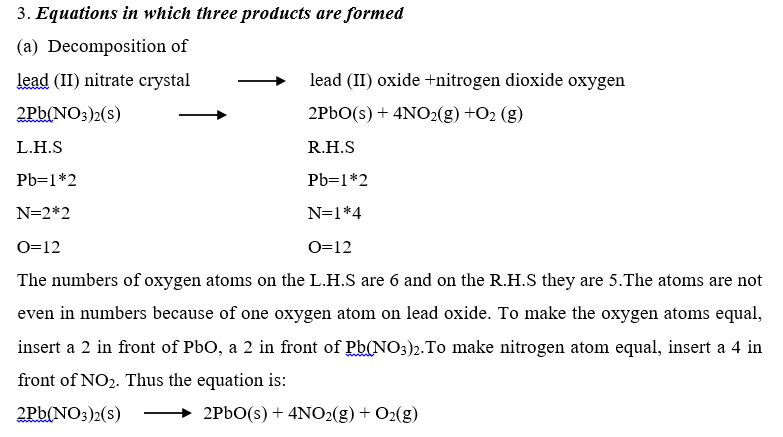
Examples on writing balanced equations.
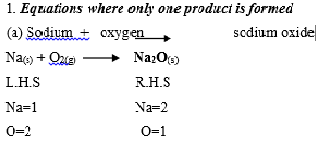
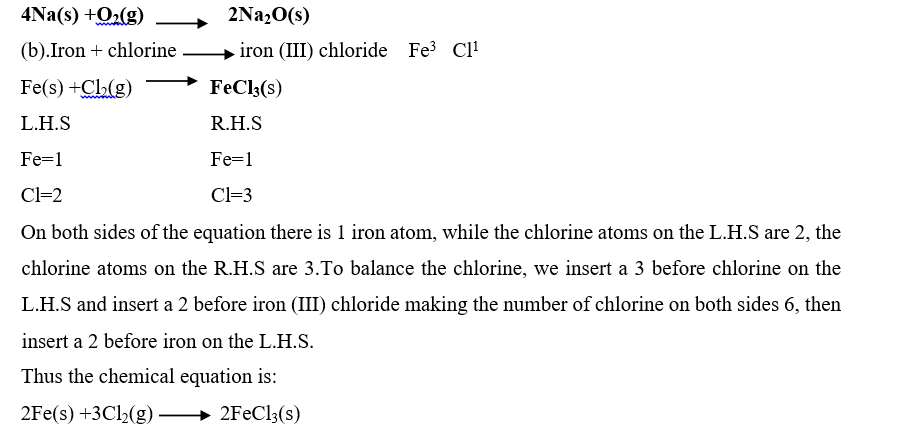
On the left-hand side of the equation, the sodium atom is one, on the R.H.S the sodium atoms are two. To balance the sodium atoms insert a 2 before sodium atom on the left. The oxygen atoms on the right are balanced by inserting a 2 before sodium oxide. But this produces 4sodium atoms On the right while the left remains with 2, therefore to balance the equation insert a 4 on sodium atom on the left.
Thus chemical equation is: